Other submissions and monitoring
IRB oversight doesn't end after obtaining approval for an initial submission. There are post-approval submissions such as problem reporting, amendments, continuing reviews, and closure requests. The IRB may also request post-approval monitoring of a study. Click on each of the corresponding accordions below to learn more about each type of post-approval submission and VCU's Post-Approval Monitoring and Education (PAM&E).
Unanticipated problem reporting
All unanticipated problems must be reported to the IRB within five working days of learning of the event. Evaluating whether an event is an unanticipated problem is the responsibility of the principal investigator.
An unanticipated problem involving risk to subjects or others (UP) is any event that meets all of the following three criteria:
- Was not anticipated or foreseen
- Was possibly, probably or definitely related to, or caused by, the research activity in the judgment of the investigator
- Suggests that the research places subjects or others at greater risk of harm (including physical, psychological, economic or social harm) than was previously known or recognized
It is important to note that only those adverse events (AEs) that meet all three criteria above are unanticipated problems that need to be reported promptly to the IRB.
Other reporting
Noncompliance in the conduct of the research must be reported to the IRB. There may also be times when a sponsor requires reporting to the IRB or there is other information the investigator feels the IRB should know, in which case a report may be submitted to the IRB.
Submitting a report in RAMS-IRB
- Log in to RAMS-IRB and open the workspace for the study needing a report
- Click the “New Report to IRB” button
- Complete a report form and upload relevant documentation
Reporting requirements for studies approved by non-VCU IRBs
Once an initial submission is approved an external, non-VCU IRB such as NCI Central, WCG IRB, Advarra or another academic IRB, the principal investigator is responsible for directly submitting all future submissions to WCG IRB, including unanticipated problems, in accordance with that IRB's policies. All unanticipated problem determinations made by an external IRB regarding a VCU research setting should subsequently be submitted in a report to the VCU IRB via the procedure described above.
For questions regarding research reviewed by external, non-VCU IRBs contact irbreliance@vcu.edu or visit the IRB reliance webpage.
Amending exempt research
Most modifications to exempt research may be done at the discretion of the principal investigator without prior IRB approval. Some changes do require IRB approval before being implemented. For a list of changes that must be submitted to the IRB for review and approval, see the conditions of approval attached to your initial exemption letter from the IRB or refer to .
Amending expedited research
All proposed changes to expedited research must be submitted for IRB review and approval prior to initiating the change.
Amending full board research
All proposed changes to full board research must be submitted for IRB review and approval prior to initiating the change. Some types of minor changes to full board studies may be reviewed in an expedited fashion by a single reviewer. This determination is made by the IRB reviewer, not the principal investigator.
Changing the Principal Investigator (PI) on a study
If you need to amend a study to change the Principal Investigator (PI) for the study, you must submit this change in an amendment in RAMS-IRB. When changing the PI on an externally-reviewed study, email irbreliance@vcu.edu for guidance.
Submitting an amendment in RAMS-IRB
- Log in to RAMS-IRB and open the workspace for the study needing an amendment
- Click the “New amendment” button
- Fill out the amendment cover sheet
- Modify the smartform
- Upload redline and clean copies of any revised documents to the study workspace
See the RAMS-IRB researcher gif guide for details on submitting an amendment.
Ongoing submissions for WCG IRB approved research
Once an initial submission is approved by WCG IRB, the principal investigator is responsible for directly submitting all future submissions to WCG IRB, including changes in research (amendments).
A copy of all submissions must be provided to the Human Research Protection Program via IRBIntake@vcu.edu, as well as to WCG IRB.
Be aware that WCG IRB updates its submission forms as much as twice yearly to improve the review of research and protection of human subjects. When preparing to submit research for review, be sure to use the most current versions of WCG IRB submission forms by referring directly to the WCG IRB forms site.
All full board research must be re-reviewed by the IRB at least annually through a continuing review process. Some, but not all, expedited research also requires continuing review. Exempt research does not require continuing review. Well in advance of an expiration date, reminders are sent to investigators prompting preparation and submission of a continuing review submission.
Submitting a continuing review in RAMS-IRB
- Log in to RAMS-IRB and open the workspace for the study needing continuing review
- Click the “New Continuing Review” button
- Complete a continuing review form and upload relevant documentation such as DSMB reports
Maintaining approval for WCG IRB approved research
Once an initial submission is approved by WCG IRB, the principal investigator is responsible for directly submitting all future submissions to WCG IRB, including:
- Changes in research (amendments)
- Unanticipated problems
- Continuing reviews
- Study closure
A copy of all submissions must be provided to the Human Research Protection Program via IRBIntake@vcu.edu, as well as to WCG IRB. WCG IRB will send continuing review reminder notices to principal investigators. Please pay close attention to the reminders. WCG IRB strictly enforces deadlines.
Be aware that WCG IRB updates its submission forms as much as twice yearly to improve the review of research and protection of human subjects. When preparing to submit research for review, be sure to use the most current versions of WCG IRB submission forms by referring directly to the WCG IRB forms site.
Status updates of IRB-approved expedited research
Expedited research that does not require ongoing continuing review must submit a status update to the IRB at least annually through RAMS-IRB. Well in advance of an expiration date, reminders are sent to investigators prompting submission of a status update.
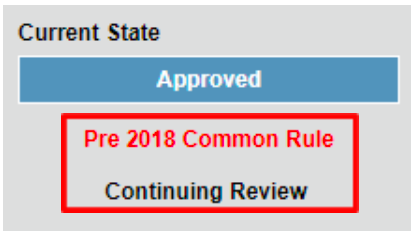
For expedited studies, investigators can tell whether their study requires continuing review by the current state listed for their study in RAMS-IRB.
- If a study requires continuing review
- If a status update is required
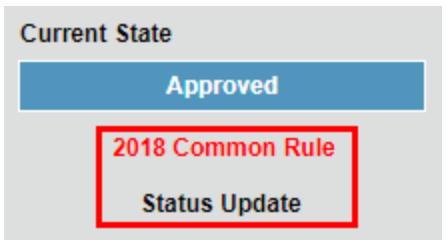
NOTE: Certain expedited studies require continuing review. If your study requires a continuing review, see accordion above titled, "Continuing review of approved IRB study."
Submitting a status update in RAMS-IRB
- Log in to RAMS-IRB (https://irb.research.vcu.edu) with your VCU eID.
Tip: Remember to connect through RAMS VPN first if you are off campus. - Go to the “My IRB Studies” tab.
Tip: If your study is expired or closed, locate it under the “Archive” tab. - Click on the title of the study for which you want to submit a continuing review.
- Click the “Submit Status Update” button.
- Complete the three to five question status update form in the pop-out window.
- Click OK to close the pop-out window and submit the status update.
You will receive an email with either:
- An automatic acknowledgement of your study’s ongoing status or
- A request to submit any necessary report or amendment to the IRB
If the issue(s) noted in the status update are not resolved with the VCU IRB prior to the expiration date, then the study will expire, and all research activities (including data analysis) must stop.
Allowing the study to expire may result in the IRB making a determination of noncompliance.
If your status update prompts you to submit a closure request
RAMS-IRB will not allow you to submit the status update if you indicate in your status update that the study is ready to close. You will be prompted to submit a closure request instead of a status update.
For more information about study closure, please review .
For instructions on how to submit a closure request, see next accordion panel, "Study close out."
If your status update prompts you to submit a report
If your status update indicates that the study has had an unanticipated problem that has not already been submitted to the IRB, you will be required to submit a report to the IRB within five working days.
The expiration date for your study will be extended by 30 days to give you time to submit the report and for the IRB to review it before the study expires.
To determine whether a problem is an unanticipated problem, the following questions should be asked:
- Is the problem unexpected?
- Is the problem related or possibly related to participation in the research?
- Does the problem suggest that the research places subjects or others at a greater risk of harm than was previously known or recognized?
If the answer to all three questions is yes, then the problem is an unanticipated problem and must be reported to the IRB immediately.
For more information about unanticipated problems, please review .
For instructions on how to submit a report, see first accordion on this page, "Problem reporting."
If your status update prompts you to submit an amendment
If your status update indicates that the study has made changes to the study protocol, procedures or study documents that have not already been approved by the VCU IRB, you will be required to submit an amendment to the IRB as soon as possible.
One of the conditions of IRB approval is that investigators must “obtain approval from the VCU IRB before implementing any changes in the approved research.” This applies for any site (VCU or non-VCU) that is under the VCU IRB’s oversight.
The expiration date for your study will be extended by 30 days to give you time to submit the amendment and for the IRB to review it before the study expires.
For more information about amendments, please review .
For instructions on how to submit an amendment, see second accordion panel on this page, "Amending an approved study."
If you filled out your study’s status update incorrectly:
Call the IRB coordinator assigned to your study before the study’s expiration date. The IRB coordinator’s name is listed in RAMS-IRB in the header information of your study, and their direct contact information is available at Contact ORSP. You may also call the IRB’s main phone number, (804) 828-0868.
When a study meets the criteria for closure, IRB oversight is no longer needed and a study may be closed with the IRB.
VCU IRB oversight may end (following a request for closure) only when ALL of the following conditions are met:
- The research is permanently closed to enrollment at the site(s) under the VCU IRB approval.
- All interactions/interventions with subjects, or access to a subject’s personally identifiable information, (including identifiable biological specimens) for the purpose of research data collection is complete.
- All use, study and/or analysis of identifiable private information at the research site(s) under the VCU IRB approval is complete.
Note regarding multicenter studies: Continuing review of the research by the VCU IRB is no longer required once the above conditions are met (following a request for closure), even if (i) interactions or interventions with subjects may be occurring at study sites other than those under the VCU IRB approval; or (ii) data analysis of identifiable private information is ongoing at another central site (site other than those under the VCU IRB approval) that collects and analyzes data from all study sites. For studies closed with the VCU IRB under these conditions, investigators may still respond to queries from the statistical center at the other institution regarding previously collected data about subjects who were enrolled under the VCU IRB approval.
As a reminder, principal investigators should retain all VCU IRB correspondence, approved documents and raw data for a minimum of five years; longer retention may be necessary based on applicable regulatory requirements and/or sponsor requirements. Refer to the VCU research data ownership, retention and access policy and the VCU Record Retention Policy for specific requirements. Data security must be ensured even after study closure if identifiable information (including coded data) is retained.
Submitting a closure request in RAMS-IRB
- Log in to RAMS-IRB and open the workspace for the study that is ready to close
- Click the “New Study Closure” button
- Complete the closure form and upload relevant documentation such as DSMB reports
Alternatively, a study can be closed at the time of continuing review by selecting the close study option within the continuing review activity.
See this RAMS-IRB researcher gif guide for details on submitting a closure request.
Maintaining approval for WCG IRB approved research
Once an initial submission is approved by WCG IRB, the principal investigator is responsible for directly submitting all future submissions to WCG IRB, including study closure requests.
A copy of all submissions must be provided to the Human Research Protection Program via IRBreliance@vcu.edu, as well as to WCG IRB.
Be aware that WCG IRB updates its submission forms as much as twice yearly to improve the review of research and protection of human subjects. When preparing to submit research for review, be sure to use the most current versions of WCG IRB submission forms by referring directly to the WCG IRB forms site.
The purpose of the Post-Approval Monitoring and Education (PAM&E) is to assist the university and investigators in conducting human research of the highest ethical quality and compliant with federal, state, and local requirements. The PAM&E team works toward this goal by improving investigator/IRB performance through monitoring, education, and measurement of overall quality, effectiveness, and efficiency. In addition, the PAM&E team acts as a resource for the VCU/VCU Health research community by offering help through problem-solving tips, templates, focused educational services, external/internal audit preparation, and referrals.
Types of visits
For cause
Visits requested by the IRB, Human Research Protection Program (HRPP) staff, institutional officials, department chairs, etc., are generally due to concerns regarding study compliance and/or research subject rights and welfare. For-cause visits may also be initiated due to complaints, repeated errors, or a lack of responsiveness by the principal investigator to IRB/HRPP requests.
Examples of the types of for-cause reviews are:
- Focused
Focused visits are more narrow reviews of research activities that concentrate on direct research subject impact. The informed consent process (including documentation and observation), recruitment, or treatment outcomes could each be the object of a focused review. This type of review is not prompted by a specific concern or issue and is similar to routine review. - Investigations
Occasionally the IRB/HRPP will request an in-depth investigation of an investigator’s studies or study conduct. This may be due to a noncompliance determination and/or significant regulatory problems found during review. This investigation may involve significant time on site reviewing study documentation and processes.
Routine
Routine visits to studies are not initiated by any cause for concern. Studies may be selected based on any number of criteria, including:
- Risk level of the study
- Studies enrolling vulnerable populations
- Studies involving investigational new drugs or devices
- Studies involving an investigator held IND-IDE
- Studies involving complex procedures
- A high number of studies conducted by a PI or managed by a study coordinator
- Random selection from all active studies
Examples of routine visits are:
- Targeted
Visits or document reviews targeted toward a particular type of study such as studies reviewed by an external IRB, expedited research, or studies involving a vulnerable population. - Educational
Visits requested by the PI, study staff, IRB, HRPP staff,department chairs, etc., to address a perceived educational need related to study conduct. This is often requested when there are new study staff/PIs as part of the onboarding process. - Preparatory review
Visits requested by the PI or research staff to assist with preparation for an external audit or in anticipation of the start of recruitment.
Overview of the PAM&E process
Most PAM&E visits follow the process outlined below. Educational visits or consultations requested by the study team may have a slightly different process.
- PAM&E contacts the investigator via email to request the visit. A mutually acceptable time is agreed upon to conduct the visit. Visits should take place within a week or two of suggested dates.
- In the visit request letter, PAM&E includes a link to or electronic version of the Post-Approval Monitoring and Quality Improvement Program Self-Evaluation Tool (PAM&E SET).
- The PI, study coordinator, and PAM&E team meet to discuss questions arising from the IRB documentation and PAM&E SET. This visit usually takes 90-120 minutes depending on questions/issues the PI and study coordinator want to discuss or the complexity of the questions that arose from the pre-visit document review.
- The PAM&E team may return on another day to observe the consent process, clinical processes, and/or complete an in-depth documentation review, etc.
- Following the visit, PAM&E will email a Study Review and CAPAeport to the PI and study coordinator noting required actions and recommendations. The required actions constitute the corrective action plan. The PI response fields on the report should be completed and returned within a week of receipt. This does not mean the required changes need to be completed within a week, but the PI response denotes the PI’s intentions and commitment to fulfilling those intentions within a stated period of time and/or before the next continuing review.
- PAM&E will upload the final report, questions and completed PI response form to RAMS-IRB for IRB review.
- If there is a possibility of the PI being cited for noncompliance (general, serious, and/or continuing), the PAM&E manager will discuss the report and PI response with the HRPP director. The findings and response will be referred to the full IRB panel if the director believes there may be noncompliance.
- If applicable and if resources are available, the PAM&E team may offer to assist the PI and study staff resolve issues, comply with the corrective action plan, and help the study move through the IRB process more smoothly.
There may be communication back and forth after the visit to clarify any additional questions that arise while the report is being written.
Preparing for a visit
Visits will be completed in a timely fashion and flow more smoothly if the following preparation is done prior to the scheduled visit:
- Complete the required pre-visit documentation and return it to PAM&E via email to pame@vcu.edu. This includes:
- Reserve a conference room with a table for the visit/record review to occur.
- Have applicable research records ready for review, including:
- IRB submissions
- Protocols
- Recruitment materials
- Subject screening and enrollment logs
- Signed consent forms
- DSMB reports
- FDA visit reports
- Communication from sponsor/funder
Education, consulting and assistance
The PAM&E team is available for education and consulting sessions, as well as assistance. PAM&E can:
- Offer recommendations for increasing compliance while maintaining or increasing good research design by helping the study team operationalize/adjust study procedures and processes to comply with federal, state, and institutional policies;
- Consult on research design and compliance; and/or
- Provide advice regarding upcoming submissions/amendments, research questions from the PI regarding design enhancements for current or upcoming research, and connect the study team with other available research resources.
Study conduct toolkit
Click here to view the study conduct toolkit, which contains a wide variety of templates to assist investigators and study coordinators with study organization and conduct.