Devices
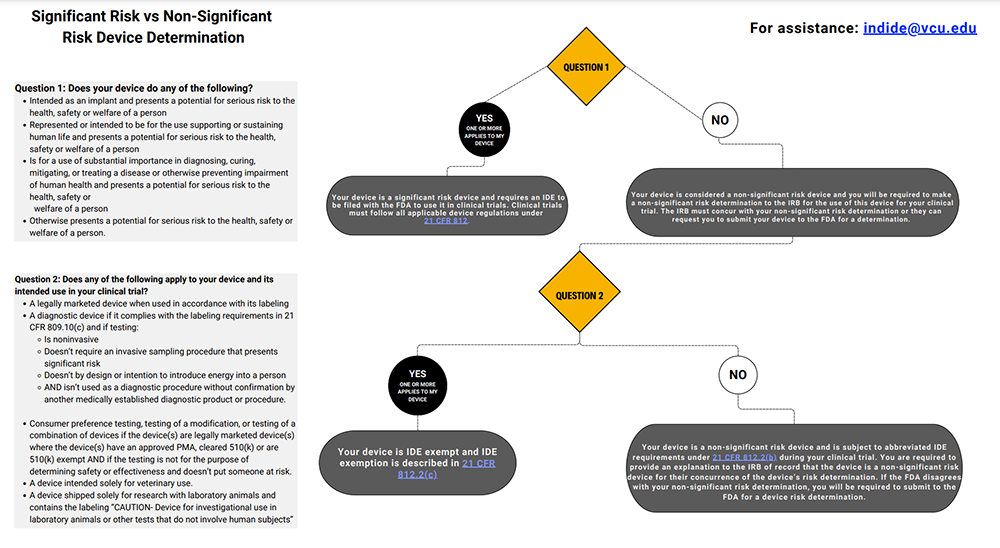 Non-significant risk vs significant risk determination decision tree (text-only version)
Non-significant risk vs significant risk determination decision tree (text-only version)
An investigational device exemption (IDE) is a submission to the FDA that allows investigations of devices to determine safety and effectiveness of devices that aren’t approved by the FDA. This allows the devices being studied to comply with any performance standards or obtain premarket approval to ship the device. IDEs are managed through the Center for Devices and Radiological Health. There are different types of IDEs subject to different regulations: “full” IDE, abbreviated IDEs, and IDE exemptions; this is dependent on the level of risk your device is and what you are planning on doing with it. For assistance on if you need an IDE or what type of IDE you would need, please contact the FDA program at VCU at indide@vcu.edu.
FDA regulations
FDA guidance and forms
- FDA device advice website
- FDA IDE website
- How to classify your device
- IDE required content and formatting
IDE general information
- Significant risk IDE sponsor and investigator responsibilities
- Non‐significant risk IDE sponsor and investigator responsibilities
IDE templates
- IDE investigator agreement for clinical investigation
- Documentation of NSR determination
- Concurrence of exemption
IDE education
On-demand investigational device exemptions sponsor and investigator training modules are available through Talent@VCU or Canvas.
- Access modules in Talent@VCU
- If you have not logged into Talent in the past 24 hours, you will need to log into talent then search for the training module “Investigational Device Exemptions (IDEs) Sponsor and Investigator Training Modules”
- Access modules in Canvas