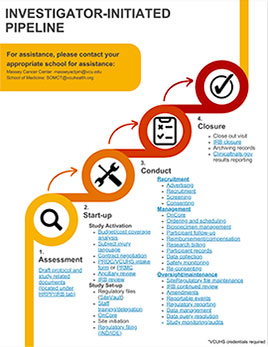
Clinical research resources
Study pipelines
Study related templates
- Delegation of authority log
- Monitoring visit log
- Concomitant medication log
- Training log
- Adverse event log
- Investigational product accountability log (dispensing to/from participant)
- Investigational product accountability log (distribution from investigational pharmacy to study team)
- Protocol deviation log
- Specimen tracking log
- Research compensation log
- Screening and enrollment log
- Corrective and Preventive Action (CAPA) Plan
VCU clinical research tools
Protocol development and services prior to initiating the clinical trial
- Intellectual property: VCU TechTransfer
- Biostatistics, Epidemiology and Research Design
- Community engagement
- Mock study sections
- Protocol development resources
- Research data management
- Cohort discovery: TriNetX, ACT and I2B2 (links may require login credentials); multisite studies: Trial Innovation Network
Budget and financial documents
Research resources prior to initiating a clinical trial
Research systems while conducting the clinical trial
- OVPRI RAMS Systems: AIRS, ECO, FORCE, IRB, SPOT and Reports
- ClinicalTrials management system: OnCore
- Electronic investigator site file and trial master file: Veeva SiteVault and Veeva eTMF
- Electronic data capture system: REDCap
Research resources during the clinical trial
- Recruitment tools:
University units or programs available for support during the clinical trial
- Clinical units: Clinical Research Unit at VCU Health and Pediatric Clinical Research Office at Children’s Hospital of Richmond
- Recruitment planning and resources
- Research hubs: Massey Cancer Center Clinical Trials Office and School of Medicine Research Administration office
- Clinicaltrials.gov program
- Researcher support available through the VCU Libraries
VCUHS services
- VCU Health System policies
It is essential to review the VCU Health policies and procedures related to clinical research, which includes essential program information, including: -
- procedures for scheduling research participants
- the VCU personnel clinical permissions registration process
- monitor access information and applicable policies and procedures to monitors of clinical trials
- resources and services available through the VCU Health Clinical Research Office to support clinical research operations and clinical trial participants.
Clinical research regulations, policies and procedures
- VCU notices and policies
- VCU compliance notices
- VCU employee and facility use guidelines for clinical research (requires VCU eID credentials)
- Federal regulations